
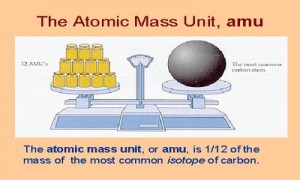
One might ask why such pains have been taken to make the distinction. Though "weigh" is used as a verb in this essay, this is only because it is less cumbersome than "measure the mass of." (In addition, "atomic weight" may be mentioned when discussing studies by scientists of the nineteenth century, who applied that term rather than atomic mass.) Indeed, the use of " atomic weight" today merely reflects the fact that scientists in the past used that expression and spoke of "weighing" atoms. The first of these is not as accurate as the second, which explains why atomic mass was chosen as the subject of this essay. Some textbooks and other sources use the term atomic weight instead of atomic mass. The measurement of atomic mass was thus a historic challenge that had to be overcome, and the story of the ways that scientists met this challenge is an intriguing one. More specifically, the work of a chemist requires the use of accurate atomic proportions in forming the molecules that make up a compound. One answer is that everything is made of atoms. But what about something so insignificant in mass that comparing it to a gram is like comparing a millimeter to the distance between Earth and the nearest galaxy? Obviously, special units are needed for such measurements then again, one might ask why it is necessary to weigh atoms at all.

Every known item of matter in the universe has some amount of mass, even if it is very small.


 0 kommentar(er)
0 kommentar(er)
